Abstract
The leukemia-associated fusion gene CBFB/MYH11 results from a pericentric inversion of chromosome 16, inv(16)(p13.1q22), or less commonly from a t(16;16)(p13.1;q22). Although this cytogenetic aberration is associated with a rather favorable prognosis in acute myeloid leukemia (AML), nearly half of patients eventually relapse after standard chemotherapy.
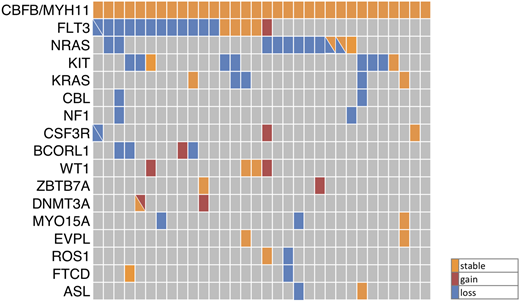
To systematically analyze the clonal evolution in this AML-subgroup, we performed whole exome sequencing (WES) of 13 adult CBFB/MYH11-rearranged AML patients using matched diagnostic, remission and relapse samples ('triplets'). Thereby, we found 2-12 (median: 8) somatic sequence variants per patient at diagnosis and 2-13 (median: 4) mutations at relapse. These included mutations in genes known to cooperate with CBFB/MYH11 (e.g. RAS, FLT3, KIT) as well as in genes, which had not been associated with AML previously (MYO15A, EVPL, ROS1, FTCD and ASL). Next, we designed a custom targeted sequencing assay (Haloplex, Agilent), including the candidate genes from exome sequencing, as well as genes known to be recurrently mutated in AML (455 genes, 1.86 Mbp total target sequence) and performed targeted sequencing of 32 CBFB/MYH11-rearranged AML triplet samples (including the 13 triplets initially analyzed by WES) with a median read depth of 500. The results are summarized in Figure 1. Fourteen genes were found mutated in at least two patients at diagnosis and 9 genes at the timepoint of relapse. In all CBFB/MYH11-rearranged patients, more than one additional mutation was identified, each of them at a distinct variant allele-frequency, indicating clonal heterogeneity. All but one FLT3 TKD (D835 or N676) mutation were lost at relapse, whereas FLT3 ITDs were stable in 3 out of 7 patients. One FLT3 ITD was gained at relapse. The majority of RAS,KIT and CBL mutations were lost and none was acquired at relapse. Particularly, the loss of 6 out of 7 KIT exon 8 frameshift mutations was surprising since KIT exon 8 frameshift mutations were negative prognostic markers in a cohort of 162 patients with CBFB/MYH11 rearranged leukemia (OS: HR= 3.12, p= 0.001; Opatz et al. submitted). In contrast, mutations in WT1 and DNMT3A were all stable during relapse evolution and four patients gained mutations in these two genes. Furthermore, aberrations in CSF3R, BCORL1 and ZBTB7A were acquired at relapse. Of note, WT1 mutations causing a frameshift in exon 6 were found in 9% of adult de novo AML with CBFB/MYH11-rearrangement and have recently been characterized by our research group as negative prognostic marker for overall survival (HR: 2.93, p= 0.011) (Opatz et al. submitted). These findings are in line with the observed gain of WT1 mutations in 10% of relapsed cytogenetically normal AML patients (Greif et al., 2018, Clin Cancer Res) suggesting a common mechanism of disease progress across cytogenetic subgroups. Surprisingly, a mutation in ZBTB7A, a gene frequently altered in RUNX1/RUNX1T1 positiv leukemia (23%) but rarely in CBFB/MYH11 positiv leukemia (2%), was gained at relapse in one patient. Mutations in epigenetic modifiers, cohesion complex components and janus kinases are known cooperating events in RUNX1/RUNX1T1 rearranged leukemia and were, except for DNMT3A, not found in our CBFB/MYH11-positive cohort. The new recurring mutations in MYO15A (n=3), ROS1 (n=2), FTCD (n=2) and ASL (n=2) were partially lost at relapse, whereas EVPL (n=2) mutations were stable during the course of disease. In addition, we identified mutations in APC2, TP53 and ZFHX4 (gained at relapse), PTPN11, MECOM, BCOR, NPM1 and IDH2 (stable) as well as in ABL1 (lost at relapse) in individual patients.
Taken together, our findings suggest that mutations in signaling pathway genes seem to be unstable during disease progression and may thus not be required for the evolution of relapse. The frequent loss of signaling gene mutations indicates that relapse might evolve from an early ancestral clone carrying the CBFB/MYH11 rearrangement only.
Figure 1: Mutation profile of 32 patients with CBFB/MYH11-rearranged AML. The stability of recurrently mutated genes during the evolution of relapse is shown. Diagonal lines indicate two mutations in the respective gene.
Thiede:AgenDix: Other: Ownership; Novartis: Honoraria, Research Funding. Middeke:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Stoelzel:Neovii: Speakers Bureau. Metzeler:Novartis: Consultancy; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal